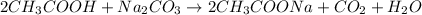Answer: Substance X is ethanoic acid and the reactions are given below.
Step-by-step explanation:
A compound having molecular formula of
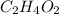 which turns blue litmus to red is considered as an acid.
which turns blue litmus to red is considered as an acid.
The compound is ethanoic acid which is generally termed as acetic acid.
Thus, the compound X is acetic acid.
The chemical equations for the reaction of X with
- a. Ethanol in the presence of
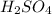
When acetic acid reacts with etahnol in the presence of an acid, it leads to the formation of a fruity smelling compound known as ester which is ethyl acetate.
![CH_3COOH+CH_3CH_2OH\xrightarrow[]{H_2SO_4}CH_3COO-CH_2CH_3+H_2O](https://img.qammunity.org/2019/formulas/chemistry/high-school/c6oo1pr36tgxqt9eecibvukrjgskev544v.png)
When acetic acid reacts with sodium carbonate, it leads to the formation of sodium acetate, carbon dioxide and water.