Answer:
The molarity of CsOH solution is 0.22 M
Step-by-step explanation:
The equation for the reaction is:
HBr + CsOH —> CsBr + H₂O
The Molarity (M) or Molar Concentration is the number of moles of solute that are dissolved in a given volume.
The Molarity of a solution is determined by the following formula:
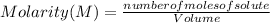
Molarity is expressed in units
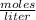
Seeing the previously presented reaction, you can see that the molar ratio between the reagents is 1. This indicates that the amount of moles must be equal, with the amount of moles M * V (volume). So: Ma * Va=Mb * Vb
In this case, being a=HBr and b=CsOH:
- Ma= 0.25 M
- Va= 26.4 mL= 0.0264 L
- Mb= ?
- Vb= 30 mL= 0.030 L
Replacing:
0.25 M* 0.0264 L= Mb* 0.030 L
Solving:
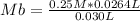
Mb=0.22 M
The molarity of CsOH solution is 0.22 M