Answer:
51 grams of ammonia would be formed.
Step-by-step explanation:
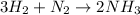
Moles of hydrogen gas = 4.50 moles
According to reaction, 3 moles of hydrogen gas gives 2 moles of ammonia gas.
Then 4.50 moles of hydrogen gas will give:
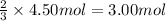 of ammonia
of ammonia
Mass of 3.00 moles of ammonia:
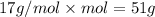
51 grams of ammonia would be formed.