Answer: The balanced equation for the above reaction is given below.
Step-by-step explanation:
Combustion reaction is defined as the reaction in which a hydrocarbon reacts with oxygen to produce carbon dioxide and water molecule.
The balanced chemical equation for the combustion of methane follows:
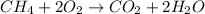
By stoichiometry of the reaction:
1 mole of methane gas reacts with 2 moles of oxygen gas to produce 1 mole of carbon dioxide and 2 moles of water molecule.
Hence, the balanced chemical equation is given above.