Answer:
![Keq =([H+]^(2) [SO4^(2-)]^(2) )/([H2SO4])](https://img.qammunity.org/2019/formulas/chemistry/college/l5e3k7ve40ftivkhsut4szpkctgxpnzia1.png)
Step-by-step explanation:
The equilibrium constant K is a parameter that relates the concentration of products to that of the reactants at equilibrium and under a given temperature.
Consider a hypothetical reaction:
xA + yB ↔ zC
where A and B are the reactants ; C is the product
x and y are the coefficients of the reactants; z is the product coefficient
The equilibrium constant is given as:
![Keq = ([C]^(z) )/([A]^(x)[B]^(y))](https://img.qammunity.org/2019/formulas/chemistry/college/s00ychsxo8wyl91ket1n9k23k4rt2ab488.png)
The given reaction is:
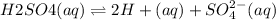
![Keq =([H+]^(2) [SO4^(2-)]^(2))/([H2SO4])](https://img.qammunity.org/2019/formulas/chemistry/college/26tv262ugjpmy7r7fkpdv29giz991yrzep.png)