Answer : The number of moles of carbon in the original sample were 0.166 moles.
Explanation :
The chemical equation for the combustion of hydrocarbon having carbon, hydrogen and oxygen follows:
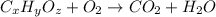
where, 'x', 'y' and 'z' are the subscripts of Carbon, hydrogen and oxygen respectively.
We are given:
Mass of
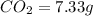
Mass of
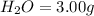
We know that:
Molar mass of carbon dioxide = 44 g/mol
For calculating the mass of carbon:
In 44 g of carbon dioxide, 12 g of carbon is contained.
So, in 7.33 g of carbon dioxide,
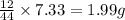 of carbon will be contained.
of carbon will be contained.
Now we have to calculate the moles of carbon.
Moles of Carbon =
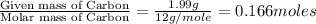
Therefore, the number of moles of carbon in the original sample were 0.166 moles.