The question is incomplete, here is the complete question:
The chemistry of fresco painting is the chemistry of limestone and lime plaster. limestone is calcium carbonate, an abundant, naturally occurring mineral. two key reactions, shown below, are involved in the process of converting limestone to lime plaster (calcium hydroxide). classify each of these reactions as one of the four types of reactions listed in this experiment (decomposition, synthesis, single replacement, or double replacement).
(a)
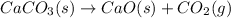
(b)
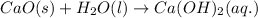
Answer: Equation (a) is a decomposition reaction and equation (b) is a combination reaction.
Explanation:
Decomposition reaction is defined as the reaction in which a single large substance breaks down into two or more smaller substances.
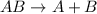
Combination reaction is defined as the reaction in which smaller substances combine to form a larger substance.
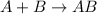
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
The reactivity of metal is determined by a series known as reactivity series. The metals lying above in the series are more reactive than the metals which lie below in the series.
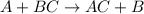
Double displacement reactions are defined as the reactions in which exchange of ions takes place.
For the given equations:
(a)
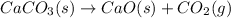
This equation is a type of decomposition reaction in which calcium carbonate breaks down into calcium oxide and carbon dioxide
(b)
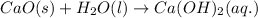
This equation is a type of combination reaction in which calcium oxide reacts with water to form calcium hydroxide.