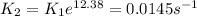Missing details. Complete text is:"The following reaction has an activation energy of 262 kJ/mol:
C4H8(g) --> 2C2h4(g)
At 600.0 K the rate constant is 6.1× 10–8 s–1. What is the value of the rate constant at 785.0 K?"
To solve the exercise, we can use Arrhenius equation:
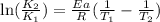
where K are the reaction rates, Ea is the activation energy, R=8.314 J/mol*K and T are the temperatures. Using T1=600 K and T2=785 K, and Ea=262 kJ/mol = 262000 J/mol, on the right side of the equation we have
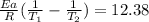
And so
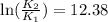
And using
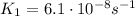
, we find K2: