Answer: The molar mass of sucrose is 342 g/mol
Step-by-step explanation:
Molar mass is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the molecular formula of a compound. It is expressed in g/mol.
The molecular formula of sucrose is
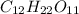
We know that:
Atomic mass of carbon = 12 g/mol
Atomic mass of hydrogen = 1 g/mol
Atomic mass of oxygen = 16 g/mol
Molar mass of sucrose =
![(12* 12)+(22* 1)+(11* 16)]=342g/mol](https://img.qammunity.org/2019/formulas/chemistry/high-school/1zc1btekah05qaujd7fg363iqw02h920bh.png)
Hence, the molar mass of sucrose is 342 g/mol