Answer : The mass of carbon present in 55.0 grams of the substance will be, 15.006 grams.
Explanation :
First we have to calculate the total mass substance.
Total mass of substance = Mass of carbon + Mass of oxygen
Total mass of substance = 2.00 g + 5.33 g = 7.33 g
Now we have to calculate the mass of carbon present in 55.0 grams of substance.
As, 7.33 grams of substance contain 2.00 grams of carbon.
So, 55.0 grams of substance contain
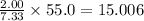 grams of carbon.
grams of carbon.
Therefore, the mass of carbon present in 55.0 grams of the substance will be, 15.006 grams.