AnswerThe required balanced reaction is given as:
Explanations
![3K_2CO_3(aq)+2Al_2\operatorname{\lparen}NO_3)_3+3H_2O\rightarrow2Al(OH)_3+3CO_2+6KNO_3]()
Given the following
Mass of potassium carbonate = 22.5grams
Determine the moles of potassium carbonate
Moles of potassium carbonate = mass/molar mass
22.5/138.205|
= 0.1628moles
Since the precipitate formed is aluminum hydroxide, hence the mole of precipitate formed if 3moles off K2CO3 produce 2moles of Al(OH)3 is given as:
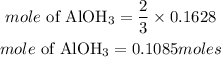
Hence the moles of dried precipitate should be formed