Answer: A) The air molecules heated inside the tire causing them to move faster exerting more pressure on the inside walls of the tires.
Step-by-step explanation: Gay-Lussac's Law: This law states that pressure is directly proportional to the temperature of the gas at constant volume and number of moles.
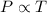 (At constant volume and number of moles)
(At constant volume and number of moles)
Thus in a tire whose volume and number of molecules is fixed, the temperature is increased, the air molecules will heat and the kinetic energy of the molecules increase. The molecules start moving faster and will collide faster with walls thus exerting greater pressure on the walls of the tires.