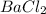Answer: The chemical formula for the given compound is
Step-by-step explanation:
Barium chloride is formed by the combination of barium and chlorine atoms. This compound is ionic compound because electrons get transferred from one atom to another atom.
Barium is the 56th element of the periodic table. It is a metal and it looses its electrons. The electronic configuration for this element is
![[Xe]6s^2](https://img.qammunity.org/2019/formulas/chemistry/college/ve56mdldv6ax1tva19hr3a7i6js1o8cu4w.png)
This element will loose 2 electrons and forms
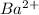 ion.
ion.
Chlorine is the 17th element of the periodic table. It is a non-metal and it gains electrons. The electronic configuration for this element is
![[Ne]3s^23p^5](https://img.qammunity.org/2019/formulas/chemistry/high-school/4hwjofwetiiiju8hlj98hs8an4c3qraui4.png)
This element will gain 1 electron and forms
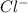 ion.
ion.
Hence, the chemical formula for the given compound is