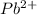Answer: The element is lead and the symbol of the ion is
Step-by-step explanation:
An ion is formed when a neutral atom looses or gains electrons.
- When an atom looses electrons, it results in the formation of positive ion known as cation.
- When an atom gains electrons, it results in the formation of negative ion known as anion.
Atomic number is defined as the number of protons or electrons that are present in a neutral atom.
Atomic number = number of protons = number of electrons
We are given:
Number of protons = 82
Number of electrons = 80
Charge = Number of protons - Number of electrons = 82 - 80 = +2
The element having 82 protons is Lead. So, the symbol of the ion is
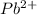 ion
ion
Hence, the element is lead and the symbol of the ion is