Answer:
c) increasing the volume by a factor of three.
Step-by-step explanation:
Hello,
By recalling the Boyle's Law:
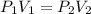
One states the pressure change:
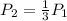
Now, replacing the pressure at the second state one obtains for the volume at the second state:
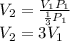
Finally, one concludes that the volume must be increased by a factor of three.
Best regards.