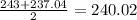Answer: B. Nickel Copper Zinc
Step-by-step explanation:
Dobereiner's triads was a group of three elements. Dobereiner said that the relative atomic mass of the middle element in each triad was average of the relative atomic masses of the other two elements.
Thus if A, B and C forms Dobereiner's triads, the mass of B is average of mass of A and C.
A. Hydrogen Oxygen Nitrogen:
mass of oxygen = 15.999
mass of oxygen according to Dobereiner =
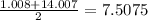
B. Nickel Copper Zinc
mass of copper = 63.5
mass of copper according to Dobereiner =
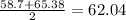
C. Aluminum Gallium Indium
mass of gallium = 69.72
mass of gallium according to Dobereiner =
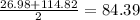
D. Neptunium Plutonium Americium
mass of Plutonium = 244
mass of Plutonium according to Dobereiner =