Answer: –2,840 kJ/mol
Step-by-step explanation:
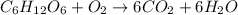
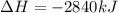
Heat of combustion is the heat produced when 1 mole of the substance is completely oxidized. The production of heat means the reaction is an exothermic process and gives off energy.
According to the given balanced chemical equation:
1 mole of glucose is being completely oxidized to give carbon dioxide and water and thus heat of combustion is -2840 kJ/mol.