Answer: The correct answer is Option b.
Step-by-step explanation:
To determine the number of electrons transferred, we need to first determine the half cell reactions.
For the given chemical reaction:
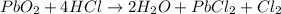
Lead is getting reduced because its oxidation state is changing from +4 to +2. Chlorine is getting oxidized because its oxidation state is changing from -1 to 0.
The half cell reactions for the above equation are:
Oxidation half reaction:
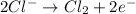
Reduction half reaction:
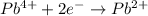
From the above half reactions, total number of electrons transferred will be two.
Hence, the correct answer is Option b.