Answer: Ionization energy increases as we move from left to right in a period.
Step-by-step explanation:
Elements are arranged in 7 periods and 18 groups in a periodic table.
Ionization energy is defined as the energy required to remove an outermost electron from from an isolated gaseous atom.
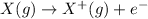
As, we move from left to right in a period, the electrons get added up into the same shell and is more attracted towards the nucleus.
This added electron requires more energy to be removed because it is attracted more towards the nucleus.
Hence, in a period, ionization energy increases.