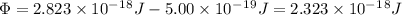The electron's energy when it leaves the metal is equal to the energy of the photon that hit it minus the energy required to break free of the metal (the work function) The photon's energy was
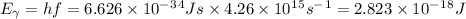
The work function is the difference between this and the electron's kinetic energy: