Answer:
9 Moles of Hydrogen atoms
Step-by-step explanation:
In order to answer this question, we can use the analogy of a motor car.
One car contains 1 steering wheel, 2 headlights and 4 tyres.
Similarly, 1 mole of
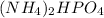 contains 9 moles of hydrogen atoms. One mole points to 6.02
contains 9 moles of hydrogen atoms. One mole points to 6.02
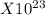 number of particles.
number of particles.
WORKING:
In
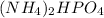 , there are 4x2 = 8 Hydrogen atoms in
, there are 4x2 = 8 Hydrogen atoms in
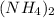 and 1 Hydrogen atom in
and 1 Hydrogen atom in
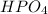 . Therefore, there are 8+1= 9 moles of Hydrogen atoms.
. Therefore, there are 8+1= 9 moles of Hydrogen atoms.