Answer: 20 gram of sample of water will have greater mass of hydrogen.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
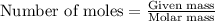 .....(1)
.....(1)
The chemical formula for water is

Given mass of water = 10 g
Molar mass of water = 18 g/mol
Putting values in equation 1, we get:
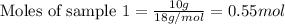
In 1 mole of water, 2 moles of hydrogen and 1 mole of oxygen atoms are present.
Moles of hydrogen =

Calculating the mass of hydrogen in 0.55 moles of water by using equation 1, we get:
Molar mass of hydrogen atom = 1 g/mol
Moles of hydrogen atom = 1.11 moles
Putting values in equation 1, we get:
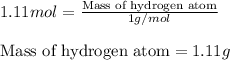
Mass of hydrogen atom = 1.11 g
Given mass of water = 20 g
Molar mass of water = 18 g/mol
Putting values in equation 1, we get:

In 1 mole of water, 2 moles of hydrogen and 1 mole of oxygen atoms are present.
Moles of hydrogen =
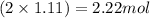
Calculating the mass of hydrogen in 1.11 moles of water by using equation 1, we get:
Molar mass of hydrogen atom = 1 g/mol
Moles of hydrogen atom = 2.22 moles
Putting values in equation 1, we get:
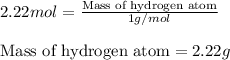
Mass of hydrogen atom = 2.22 g
Hence. 20 gram of sample of water will have greater mass of hydrogen.