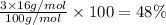Step-by-step explanation:
Percentage of an element in a compound:
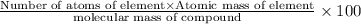
Mass of magnesium atom = 24 g/mol
Mass of silicon atom = 28 g/mol
Mass of oxygen atom = 16 g/mol
Molar mass of
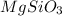 =24 g/mol+28 g/mol+3 × 16 g/mol=100 g/mol
=24 g/mol+28 g/mol+3 × 16 g/mol=100 g/mol
Composition of all elements in enstatite,
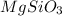
Percentage of magnesium :
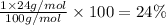
Percentage of silicon:
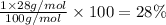
Percentage of oxygen: