Answer: The correct answer is option 2.
Step-by-step explanation:
There are 4 types of decay process, in which a radioisotope can become stable. They are:
1.) Alpha decay: In this process, alpha particles are emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units and has a mass of 4 units. The alpha particle released is also known as helium atom.
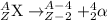
2.) Beta-decay: In this process, a neutron is converted into a proton and an electron with the release of a beta-particle. The beta particle released carries a charge of -1 units. and it does not have any mass.
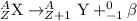
3.) Gamma ray emission: In this process, an unstable nuclei releases excess energy by a spontaneous electromagnetic process and
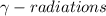 are emitted. These radiations does not carry any charge and are electrically neutral.
are emitted. These radiations does not carry any charge and are electrically neutral.
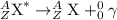
4.) Positron decay: In this process, a proton is converted to a neutron and an electron neutrino and positron particles are released. This particle carries a charge of +1 units and does not have any mass. This type of decay is also known as beta-plus decay.
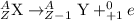
From the above information, gamma decay is represented as
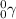
Hence, the correct answer is Option 2.