Answer :
Part 15 : Only gold contains
 number of particles.
number of particles.
Part 16 : The correct option is, (a) the formula of the compound and the atomic mass of its elements.
Explanation :
Part 15 :
As we know that, 1 mole of substance occupies 22.4 L volume of gas and 1 mole of substance contains
 number of particles.
number of particles.
So, 22.4 L volume of gas contains
 number of particles.
number of particles.
(a) Gold :
Gold contains
 number of particles.
number of particles.
(b) Sodium chloride (NaCl) :
In sodium chloride, there are 2 atoms. So, it contains
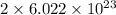 number of particles.
number of particles.
(c) Sulfur (S₈) :
In sulfur, there are 8 atoms. So, it contains
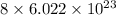 number of particles.
number of particles.
(d) Carbon dioxide (CO₂) :
In carbon dioxide, there are 3 atoms. So, it contains
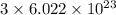 number of particles.
number of particles.
Hence, from this we conclude that only gold contains
 number of particles.
number of particles.
Part 16 :
Percent composition : It is calculated by dividing the mass of each element in one mole of the compound that means dividing the mass of each element by the total molar mass of the compound.
Formula used :
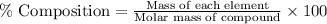
Hence, the information needed to calculate the percent composition of a compound is, the formula of the compound and the atomic mass of its elements.