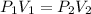The given equation is
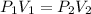 , illustrated and derived through the Boyle’s law.
, illustrated and derived through the Boyle’s law.
Explanation:
Boyle’s law derived the conditions of the sample of a gas in two different conditional pressure and volume. The initial observation was that the product of a given pressure and volume for the sample of gas at a constant temperature is observed to be constant.
Therefore,
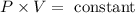
The two temperature conditions were thus observed and the equation was elaborated to be,