Answer: 6.Step-by-step explanation:1) Aluminum

So each atom of aluminum lost 3 electrons to pass from 0 oxidation state to 3+ oxidation state.
2) Manganesium
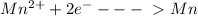
So, each ion of Mn(2+) gained 2 electrons pass from 2+ oxidation state to 0.
3) Balance
Multiply aluminum half-reaction (oxidation) by 2 and multiply manganesium half-raction (reduction) by 3:
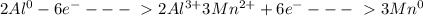
4) Net equation
Add the two half-equations:
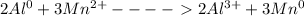
As you see the left side has 2 Al, 3Mn, and 3*2 positive charges.
The right side has 2 Al, 3 Mn, and 2*3 positive charges.
So, the equation is balanced.
5) Count the number of electrons involved.
As you see 2 atoms of aluminum lost 6 electrons (3 each).
That is the answer to the question. 6 electrons will be lost.