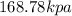Answer:
The pressure of 0.5 mole of nitrogen gas=
Given:
Moles of nitrogen gas=0.5mole
Volume of nitrogen gas=5L
Temperature of nitrogen gas=203K
To Find:
Pressure of 0.5g mole of nitrogen gas
Step by Step by Explanation:
Formula used for calculating the pressure of a gas is achieved through ideal gas equation
According to Ideal Gas;
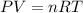
From this above equation, pressure can be calculated as
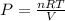
Where P=Pressure of the gas
N=number of moles=0.5moles
R=Gas constant
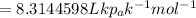
V=Volume of nitrogen gas in litres=5L
T=Temperature of nitrogen gas=203K
Substitute all the values in the above equation we get
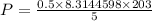
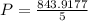
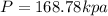
Result:
Thus the pressure of 0.5 mole of nitrogen gas is