Answer : The missing coefficient in the balanced equation is, 1 and this is a type of combustion reaction.
Explanation :
Law of conservation of matter : It states that the number of atoms of each element present of the reactant side must be equal to the product side.
The given chemical reaction is,
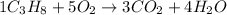
The given chemical reaction is balanced.
By the stoichiometry we can say that, 1 moles of propane react with 5 moles of oxygen to give 3 moles of carbon dioxide and 4 moles of water.
The given reaction is a combustion reaction.
Combustion reaction : it is a reaction in which the a hydrocarbon react with the oxygen to give carbon dioxide and water as a product.
Hence, the missing coefficient in the balanced equation is, 1 and this is a type of combustion reaction.