Answer:
alpha particle
Step-by-step explanation:
In a process where alpha particle is emitted, a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
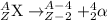
The atomic mass of the resulting lighter nuclei reduces by 4.
The process in which beta particle is emitted, a neutron gets converted into a proton and an electron releasing a beta-particle. The beta particle released carries a charge of -1 units.
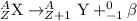
The mass of the resultant nuclei remains same.
The process in which a proton gets converted to neutron and an electron neutrino, a positron is released. This particle carries a charge of +1 units.
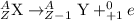
The mass of the resultant nuclei remains unchanged.
In gamma ray emission,
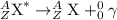
A photon is emitted. An unstable nuclei gets stabilized by release of photon. There is no change in the nuclei.
Thus, in a reaction of decay of a large atom, release of alpha particle would result in substantial change in atomic's mass.