Answer:
A.0.10 HBr
Step-by-step explanation:
We are given that some solutions
We have to find the solution which have lowest pH
1.0.10 m HBr
We know that
![p^H=-[log H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/r20geflhwg59y9qubj5tagfgx6qeqy1tz5.png)
[H+]=0.10=
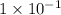
![pH=-[log(1* 10^(-1))]](https://img.qammunity.org/2019/formulas/chemistry/high-school/tg91rh7e9uf7c3o8sd5r7lfxc2tndv2xrn.png)
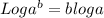
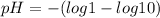
We know that log 1=0 abd log 10=1
Then we get
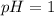
HBr is strongest acid ,therefore, it completely ionize into its ions.
But HF and
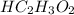 are weaker acids and do not ionize completely into its ions.
are weaker acids and do not ionize completely into its ions.
The pH of these two acids are greater than one.
Hence, the pH of HBr is lowest.
Option A is true.