Answer : The mass of sodium chloride present in 4.20 mole of NaCl are 245.7 grams.
Explanation : Given,
Moles of sodium chloride (NaCl) = 4.20 mole
Molar mass of sodium chloride (NaCl) = 58.5 g/mole
Formula used :
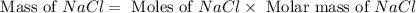
Now put all the given values in this formula, we get the mass of sodium chloride.
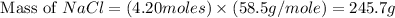
Therefore, the mass of sodium chloride present in 4.20 mole of NaCl are 245.7 grams.