Answer:
3.08 m
Step-by-step explanation:
To calculate the molarity, you have to use the following formula:
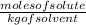
From the question, we already have the mass of the solvent (benzene); However, for the calculation of molality, we require the solvent in Kg, therefore we do the conversion from g to kg:
125 g of benzene = 0.125 kg of benzene.
To obtain the number of moles of solute we have to use the molecular weight of toluene, which is 92.14
To convert from g to moles, we divide the grams of toluene by the molecular weight:
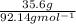
Therefore, we have 0.386 moles of toluene.
The last step will be to replace all the values in the molality formula:
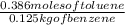 = 3.08 m
= 3.08 m
I hope this clarifies your inquiry.