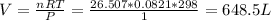Answer:
Volume of O2 = 649 L
Step-by-step explanation:
Given data:
Volume of butane, V = 100 L
Temperature , T = 25 C = 25 + 273 = 298 K
Standard Pressure, P = 1 atm
Step 1: Calculate the moles of butane using ideal gas equation
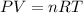
where n = number of moles, R = 0.0821 Latm/mol-K
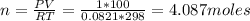
Step 2: Calculate moles of O2 required from Reaction stoichiometry
2 C₄H₁₀(g) + 13 O₂(g) ⇄ 8 CO₂(g) + 10H₂O(l)
Based on the stoichiometry:
2 moles of butane reacts with 13 moles of O2
Therefore, 4.087 moles of butane will react with:
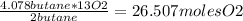
Step 3: Calculate the volume of O2 required
Again from ideal gas equation: