Answer: The correct option is a.
Explanation: We are given a balanced chemical equation for an acid-base reaction:

We need to find the concentration of base when it is neutralized by an acid.
For the neutralization reaction, we use the formula:
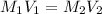
where,
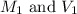 are the molarity and volume of acid.
are the molarity and volume of acid.
and
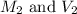 are the molarity and volume of base.
are the molarity and volume of base.
In the given question,
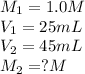
Putting all the values, in above equation, we get
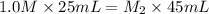
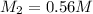
Hence, the correct option is a.) 0.56M NaOH.