Answer: Option (a) is the correct answer.
Step-by-step explanation:
A strong acid is an acid which completely dissociates into its ions in a solution.
For example, HCl is a strong acid and it dissociates as follows.
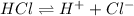
Whereas a weak acid dissociates slightly into a solution.
Thus, we can conclude that it is true of a strong acid that it ionizes close to 100 percent in water.