Answer:
The pH of the buffer solution is 2.78.
Step-by-step explanation:
Concentration of salt = 0.623 M
Concentration of acid = 1.41 M
Dissociation constant of acid =
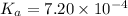
The pH of the buffer solution is given by Henderson-Hasselbalch equation:
![pH=pK_a+\log ([salt])/([acid])](https://img.qammunity.org/2019/formulas/chemistry/college/c9ovrxzjc6ow7a32zbjk5wb2o1knh1cuya.png)
![pH=-\log[K_a]+\log (salt)/(acid)](https://img.qammunity.org/2019/formulas/chemistry/college/q26wrvuc89rxytrcipvodskvrdk0uputg8.png)
![pH=-\log[7.20* 10^(-4)]+\log(0.623 M)/(1.41 M)](https://img.qammunity.org/2019/formulas/chemistry/college/5bpjr3bu0pks2isadk6w9qalj1mtf9kq0g.png)
pH = 2.78
The pH of the buffer solution is 2.78.