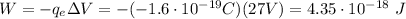1) First of all, let's calculate the potential difference between the initial point (infinite) and the final point (d=0.529x10-10 m) of the electron.
This is given by:
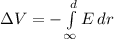
Where E is the electric field generated by the proton, which is

where
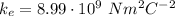
is the Coulomb constant and
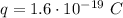
is the proton charge.
Replacing the electric field formula inside the integral, we obtain
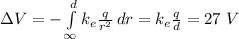
2) Then, we can calculate the work done by the electric field to move the electron (charge
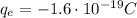
) through this
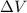
. The work is given by