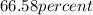Given, mass of the silicon = 6.93 g
mass of the oxygen = 7.89 g
Calculate the number of moles of both atoms:
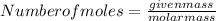
Insert the given values of mass in above formula:
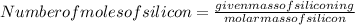
=
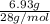
=
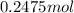
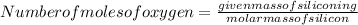
=
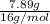
=
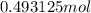
Thus,
Total number of moles = number of silicon+ number of mole of oxygen
=
 =
=
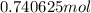
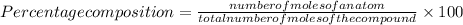
Put the values in above formula:
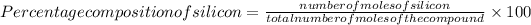
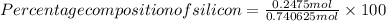
=
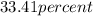
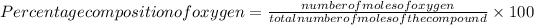
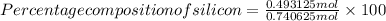
=
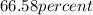
Thus, percent composition of silicon and oxygen is
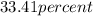 and
and