We have that all (ideal) gases obey the fundamental gas equation: PV=nRT where P is the Pressure, V is the Volume, n is the number of moles, R is a universal constant and T is the temperature in Kelvin. In this process, we have that both the number of moles and the temperature stays the same. So if we denote by i the initial conditions and by f the final conditions of the gas, we have:
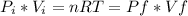
. Hence, if we solve for the final Volume we get:
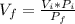
. Now we know all the other variables; substituting we get that the final volume is 6.7 L (6.716 L ).