Answer:
9 kilojoules of energy are stored in a kilogram of concrete due to sensible heat.
Step-by-step explanation:
In this exercise we need to determined the sensible heat store in a kilogram of concrete due to a change in temperature. Sensible heat (
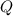 ), measured in kilojoules, is defined by the following expression:
), measured in kilojoules, is defined by the following expression:
 (1)
(1)
Where:
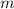 - Mass, measured in kilograms.
- Mass, measured in kilograms.
 - Average specific heat of concrete, measured in kilojoules per kilogram-degree Celsius.
- Average specific heat of concrete, measured in kilojoules per kilogram-degree Celsius.
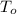 ,
,
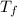 - Initial and final temperatures of concrete, measured in degrees Celsius.
- Initial and final temperatures of concrete, measured in degrees Celsius.
If we know that
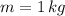 ,
,
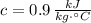 ,
,
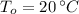 and
and
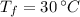 , then the energy store in a kilogram of concrete is:
, then the energy store in a kilogram of concrete is:
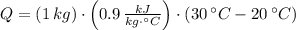
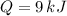
9 kilojoules of energy are stored in a kilogram of concrete due to sensible heat.