We have that after 1 half-life, the reactant reaches half its concentration
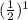
. After 2 half-lives it reaches
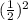
of its concentration, namely one-fourth. After n half lives we have that it reaches
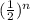
of its initial concentration. Thus, we have to solve the equation
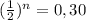
. Taking the log base 2 of each part of the equation we get from logarithm properties:
![log_(2)[(1/2)^n]=log_(2)(2^(-n))=-n](https://img.qammunity.org/2019/formulas/chemistry/college/jw932v08zpa9vj3qme54wv2rv7pa09irun.png)
. The other hand yields:
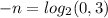
. Solving for n we get that n=1.74. Thus, after around 1.74 half lives the concentration becomes 30% of the initial one. Note how this is consistent with our previous analysis; if we let it a little bit more, 2 half-lives, the concentration will become 25% of the initial.