The quantity of heat (i.e. energy) needed to change the temperature of a substance by a certain

is
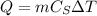
where m is the mass of the block of ice, m=500 g=0.5 Kg,
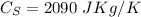
is its specific heat, and
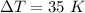
. Using these data, we obtain
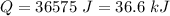
and this is the energy we must remove.