Missing data in the text of the exercise: The molar concentration of Zinc is 10 times the molar concentration of copper.
Solution:
1) First of all, let's calculate the standard electrode potential difference at standard temperature. This is given by:
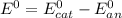
where
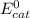
is the standard potential at the cathode, while
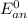
is the standard potential at the anode. For a Daniel Cell, at the cathode we have copper:
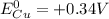
, while at the anode we have zinc:
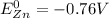
. Therefore, at standard temperature the electrode potential difference of the Daniel Cell is
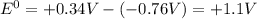
2) To calculate
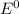
at any temperature T, we should use Nerst equation:
![E^0(T)=E^0- (R T)/(z F) \ln ([Zn])/([Cu])](https://img.qammunity.org/2019/formulas/physics/college/og15h9kyb8if50jfkc2ecr373q9wi0zovx.png)
where
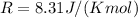
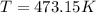
is the temperature in our problem
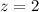
is the number of electrons transferred in the cell's reaction
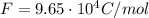
is the Faraday's constant
![[Zn]](https://img.qammunity.org/2019/formulas/physics/college/bzawkltpi986be6z28adfpd5ai7xsub3i9.png)
and
![[Cu]](https://img.qammunity.org/2019/formulas/physics/college/du7427hp0mk2f0xx5wpcyirht83fzl77fw.png)
are the molar concentrations of zinc and in copper, and in our problem we have
![[Zn]=10[Cu]](https://img.qammunity.org/2019/formulas/physics/college/vf2rcvlxxtuzddqx6h6bij27tmf4oyaq6x.png)
.
Using all these data inside the equation, and using
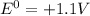
, in the end we find:
![E^0(T)=E^0- (R T)/(z F) \ln ([Zn])/([Cu])=+1.053 V](https://img.qammunity.org/2019/formulas/physics/college/5j813675tqzvp9ogpdwbxvqoqkg9p4zflb.png)