Answer: The mole ratio of
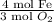 is correct for the given chemical equation.
is correct for the given chemical equation.
Step-by-step explanation:
Mole ratio is defined as the ratio of number of moles of the two substances taken into account.
In a chemical reaction, the stoichiometric coefficients represents the number of moles.
For a given chemical equation:
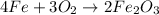
By stoichiometry of the reaction:
4 moles of iron metal reacts with 3 moles of oxygen gas to produce 2 moles of iron (III) oxide
Hence, the mole ratio of
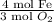 is correct for the given chemical equation.
is correct for the given chemical equation.