Answer:
There are 1600 atoms when organism just died.
Step-by-step explanation:
The statement is incorrect. The correct statement is:
If a sample known to be about 11,460 years old and has 400 carbon 14 atoms. How many atoms are in the sample when organisms just died?
The amount of atoms associated with radioactive isotopes decreases exponentially in time by means of the following formula:
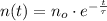 (1)
(1)
Where:
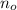 - Initial amount of atoms.
- Initial amount of atoms.
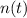 - Current amount of atoms.
- Current amount of atoms.
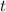 - Time, measured in years.
- Time, measured in years.
 - Time constant, measured in years.
- Time constant, measured in years.
In addition, the time constant can be calculated in terms of the half-life of the radioactive isotope (
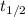 ), measured in years:
), measured in years:
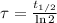 (2)
(2)
If we know that
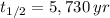 ,
,
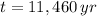 and
and
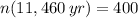 , then the initial amount of atoms is:
, then the initial amount of atoms is:
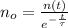
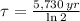
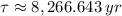
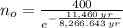
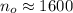
There are 1600 atoms when organism just died.