Step-by-step explanation:
Nuclear reactions are the reactions in which nucleus of an atom changes either by splitting or joining with the nucleus of another atom.
There are two types of nuclear reactions.
- Nuclear fission - In this process, large atomic nuclei splits into smaller nuclei.
- Nuclear fusion - In this process, two small nuclei combine together to form a large nuclei.
Both nuclear fission and fusion processes involve nuclei of atoms.
For example,
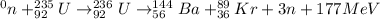
Thus, we can conclude that statements which are true are as follows.
- Nuclear reactions involve the nuclei of atoms.
- The products of nuclear reactions are lighter than the reactants.