Answer: Option (c) is the correct answer.
Step-by-step explanation:
When there are same number of protons but different number of neutrons in the nuclei of two or more forms of an atom then they are known as isotopes.
For example,
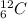 and
and
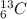 are isotopes.
are isotopes.
Thus, we can conclude that out of the following options, isotopes are defined as forms of the same element that differ in the number of neutrons in each atom.