Answer : A tetrahedral molecule has 4 regions of high electron density around the central atom. These molecules have central atoms with 0 lone pairs and 4 atoms bonded to them.
Explanation :
As we are given the molecular geometry of the molecule that is tetrahedral and the hybridization of tetrahedral molecule is,
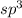 .
.
That means the number of electron density around the central atom is 4 and the electronic geometry or the molecular geometry of the molecule will be tetrahedral.
And these molecule have central atoms with zero (0) lone pair and four (4) atoms bonded to them.
For example : In
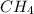 molecule, the central atom is carbon and neighboring atoms are 4 hydrogen atoms. The number of electron density is 4 that means the hybridization will be
molecule, the central atom is carbon and neighboring atoms are 4 hydrogen atoms. The number of electron density is 4 that means the hybridization will be
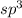 and the electronic geometry or the molecular geometry of the molecule will be tetrahedral.
and the electronic geometry or the molecular geometry of the molecule will be tetrahedral.
In
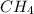 molecule, the central atom (carbon) has zero lone pair and the central atom (carbon) bonded with 4 hydrogen atoms.
molecule, the central atom (carbon) has zero lone pair and the central atom (carbon) bonded with 4 hydrogen atoms.