Answer:
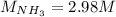
Step-by-step explanation:
Hello,
By using the following formula, one could compute the required molarity of the neutralized solution of ammonia since in the neutralization point the both acid's and base's moles must be equal as long as in the reaction a 1-to-1 mole relationship is present between them:
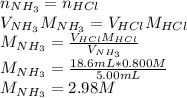
Best regards.